METHODS
(Edited excerpt from Kořínek V. 1999. A guide to limnetic species of Cladocera of African inland waters (Crustacea, Branchiopoda). Occasional Publications 1, 57pp.The International Association of Theoretical and Applied Limnology, BLT Geneva).
1. Collecting Cladocera

 It is easy to collect cladocerans in small habitats where a boat is not necessary. A small dip net on a long handle (at least 2 m long, telescopic), or a throwing net with a line about 10 m long, both of them of 0.1 mm mesh size and with a mouth diameter of 15 to 20 cm, are quite satisfactory. In larger lakes or reservoirs larger nets are used and special methods for collecting zooplankton are mentioned in the limnological literature. It is important to collect enough material, as some of the species are not numerous. Any good identification cannot be based on only one or a few individuals. Always a large population has to be examined in order to get reliable results. In some cases, more specimens are needed for dissection in order to see some of the diagnostic characters. Furthermore, it is a good idea to keep your own collection of permanent mounts of the species (in addition to the voucher collection of whole specimens) that are repeatedly encountered in local limnological studies, as comparative material.
It is easy to collect cladocerans in small habitats where a boat is not necessary. A small dip net on a long handle (at least 2 m long, telescopic), or a throwing net with a line about 10 m long, both of them of 0.1 mm mesh size and with a mouth diameter of 15 to 20 cm, are quite satisfactory. In larger lakes or reservoirs larger nets are used and special methods for collecting zooplankton are mentioned in the limnological literature. It is important to collect enough material, as some of the species are not numerous. Any good identification cannot be based on only one or a few individuals. Always a large population has to be examined in order to get reliable results. In some cases, more specimens are needed for dissection in order to see some of the diagnostic characters. Furthermore, it is a good idea to keep your own collection of permanent mounts of the species (in addition to the voucher collection of whole specimens) that are repeatedly encountered in local limnological studies, as comparative material.
2. Preservation of cladoceran samples
There are two routine ways to preserve zooplankton for further identification and treatment. Either 40 % formaldehyde solution is added to the total volume of the sample to get a final dilution of about 4 % formaldehyde, or the zooplankton is filtered out and transferred to 70 % ethyl alcohol (ethanol). The first method is preferable as small deviations to slightly lower or higher dilutions are not dangerous. Using alcohol, the results always depend on the total replacement of lake water with preservative and it is a good practice to replace the alcohol several times in order to get a final 70 %, or even higher, concentration. Preferably the content of the net should be screened through a small sieve and then washed down with ethanol into the sampling vial. Because dehydration of the material in alcohol is fast, some of the sensitive species with thin cuticles may be distorted. Samples containing alcohol have to be kept in vials with tight caps to reduce evaporation to a minimum. The danger of samples drying out is, however, always present.

On the other hand, a formaldehyde solution is stable, preserves the material well, and evaporation of water from sampling vials or bottles is slow. It is important, for both these methods of preservation, to add the preservative immediately (!) on the spot, when sampling. It is detrimental to the quality of the material if the concentrated zooplankton is kept alive for any extended period. Many individuals will be dead; some of them already partly decomposed, or distorted, by the time of preservation. The danger is greater when the weather is hot and biological processes within the collecting bottle very rapid. There are of course other methods of preservation for special treatments that are mentioned in the relevant literature.
The sample should be labelled immediately. There are several possibilities for labelling the sampling vial or bottle but the only criterion is the permanency of the written label. I recommend the use of paper that is not permeable to water (tracing or plastic) and a soft pencil to write the necessary data. A label within the sampling bottle is more secure than one outside. Such a label should contain, minimally, the information on the number of the sample (a cross-reference to a field notebook), date and location. That information and other relevant biological or chemical data should also be immediately recorded in a field notebook or another recording facility.
3. Storage of samples
With the exception of museums, it is not a commonly accepted idea to store the zooplankton material collected during a research project for any extended period. Considering the labour and the financial support involved in the collecting, it is a pity that after a decade almost no material is available for either comparison or other types of study. Nevertheless, zooplankton samples are stored in depository rooms in some scientific institutions. For such situations, the original samples have to be adjusted. First, it is usually necessary to concentrate the sample to a smaller volume eg. to vials of 10 to 40 ml capacities. It is preferable to transfer material preserved in formalin to 70% alcohol since long storage in the former preservative makes the specimens brittle; they hang together and are not easy to dissect apart. The possibility of the formation of paraldehyde sediment is also high. The addition of 10% by volume of glycerol or propylene glycol to each ethanol sample is a good precaution against drying. The vials should be closed with plastic stoppers and it is recommended that the lids should be pierced several times with a needle so that, when the environmental temperature changes, the difference in pressure inside and outside of the vials is readily balanced and the vials do not open. All the samples from one project should then be immersed in 70% alcohol in one large bottle that has a well-sealed cap. In this way the material can be kept for decades with no necessity for frequent checks.
4. Permanent mounts
Permanent microscope slides are very helpful for future identification of similar material. There are various methods of preparation, mounting media and stains on offer and here I have selected only a few that have been used successfully in our laboratory for more than 30 years. For whole mounts, water immiscible mounting media such as Canada balsam or synthetic resins are recommended. For the dissection, it is possible to work within a drop of water miscible media like glycerol, polyvinyl alcohol (PVA) or hydro-matrix. Nevertheless, for both these media, a pre-treatment of the material is necessary prior to mounting.
Pre-treatment of the material:
1. - Individual specimens picked out from the sample should be transferred to a small volume of 70 % alcohol to which several drops of lignin pink and chlorazol black E have been added. Both these dyes should be stored as saturated solutions in 70% ethanol. Staining of the specimens before mounting is of course not essential but, unfortunately, clearing of the tissues in some mounting media is so powerful that most of the fine structures are lost for microscopic examination if they are not stained. Lignin pink is a red stain readily adsorbed to inner crustacean tissues. Chlorazol black E stains chitinous structures in blue. Both stains can be mixed in various desirable ratios according to the material and purpose selected; and stored for a long time with occasional additions of the solvent.
The time necessary for the staining depends on several variables. Different species or genera absorb the stains differently. For instance, the genus Diaphanosoma and copepods are stained very rapidly with chlorazol black while lignin pink works much more slowly. Specimens stored for many decades in museum collections or poorly preserved ones accept both the stains reluctantly.
2. - After staining, cladoceran specimens should be transferred either to:
A. a mixture of ethanol and glycerol (3:1), and then possibly dissected and mounted in any water miscible medium.
or
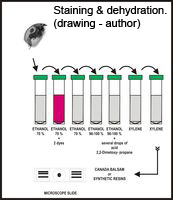 B. Whenever high quality permanent mounts are needed, of either dissected or whole specimens, Canada balsam, as a traditional medium, or synthetic resins are recommended. An easy method for dehydration of individual specimens, avoiding all the intermediate steps, is to replace 70 % ethanol with a higher concentration (96 – 100 %) and then add a few drops of a mixture of 2-2-Dimetoxypropan (DMP) and 0.2 n Chloric acid (1 ml of DMP and 1 drop of HCl). Final replacement twice with xylene is then secure - no clouding.
B. Whenever high quality permanent mounts are needed, of either dissected or whole specimens, Canada balsam, as a traditional medium, or synthetic resins are recommended. An easy method for dehydration of individual specimens, avoiding all the intermediate steps, is to replace 70 % ethanol with a higher concentration (96 – 100 %) and then add a few drops of a mixture of 2-2-Dimetoxypropan (DMP) and 0.2 n Chloric acid (1 ml of DMP and 1 drop of HCl). Final replacement twice with xylene is then secure - no clouding.
Drying of the media takes several days in Canada balsam. It is therefore better to store mounts in a horizontal position until they are well hardened inside, otherwise the specimens slowly drift to one side of the cover slip. To accelerate the drying procedure, a hot plate is recommended but the temperature must be kept within a narrow range between 40 and 50 o C.
For detailed studies of cuticular structures such as setae, spines or pores, I recommend previous heating of specimens to be stained in either concentrated lactic acid or a 10% solution of potassium hydroxide. The time of heating in a water bath is difficult to ascertain as different species and length of storage in preservative influence the destructibility of the inner tissues. It is therefore advisable to check the gradual progress of dissolution of inner tissues under the microscope. The remaining chitinous skeleton must be washed several times in distilled water to remove the alkaline or acid remnants.
If dissection is necessary, in order to see, for instance, details of thoracic limbs, the specimen should be placed in a small amount of the mounting medium and dissected directly on the microscope slide with fine needles. Needles can be prepared from thin pins or short pieces of tungsten wire that are sharpened on a whetstone to get a very thin point. The resulting pins should be fixed to a handle.
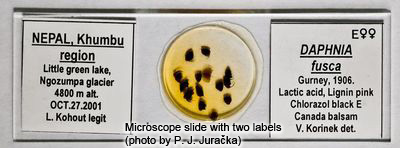
 Careful labelling of slides is important. Both the free ends of a slide can be used. On one label, the name of the species, sex, the method of staining, mounting medium and the author of the identification should be written. The other label should contain all information about the locality such as country, region, and name of the water body (including the type of habitat, such as lake, pond, pool, or ditch), date of sampling and a name of the collector.
Careful labelling of slides is important. Both the free ends of a slide can be used. On one label, the name of the species, sex, the method of staining, mounting medium and the author of the identification should be written. The other label should contain all information about the locality such as country, region, and name of the water body (including the type of habitat, such as lake, pond, pool, or ditch), date of sampling and a name of the collector.
Information should be written on an adhesive paper label.